Recently we have been queried about the relationship of insulin antibodies (IA) and the development of either hypoglycemia or hyperglycemia in various persons with type 1 diabetes mellitus (T1DM). As opposed to IA, which are induced by any type of exogenous insulin, insulin autoantibodies (IAA) occur spontaneously in the plasma of some patients (predominantly children) prior to the diagnosis of T1DM and prior to exposure to exogenous insulin.1,2 In general, IA are in much higher concentration than IAA.
A not uncommon inquiry is as follows: “A patient with T1DM is suffering recurrent and possibly severe hypoglycemia. Could this be due to insulin autoantibodies and/or the ‘insulin autoimmune syndrome’ (IAS; also known as Hirata disease)?”3 The other question is: “A patient with T1DM is taking his insulin but is in poor metabolic control. Could he be resistant to injected insulin? Should we measure his insulin levels?”
In terms of hypoglycemia that required hospital admission, often insulin and C-peptide were measured at the time of the hypoglycemic episode. Insulin present in the circulation could be endogenous or exogenous. However, the only source of C-peptide is the patient’s own pancreatic beta cells. So how are the C-peptide and insulin measurements interpreted in such clinical scenarios?
Insulin measurements
First, let’s address the question of the insulin measurement. Measuring insulin in insulin-treated patients is conceptually complex because many patients treated with insulin injections develop IA.4 This is true regardless of the type of insulin injected. Recombinant DNA insulins appear to be more immunogenic than animal insulins.5 Even exogenous human insulin is immunogenic.6 The literature reports IA frequencies of 78 percent to 97 percent in insulin-treated patients.5,7,8 IA generally raise total insulin levels because IA act as binding proteins and the insulin immunoassays measure both bound and free insulin. The problem is that it is the “free” (unbound) insulin that is believed to be biologically active. Total insulin levels in IA-positive patients do not reflect the free insulin levels. Another consideration is the degree to which proinsulin is recognized in the insulin assay.
Normally insulin has no binding proteins in the circulation, so the “total” insulin concentration in the blood is identical to “bioactive” insulin. Unlike insulin-like growth factor I (IGF-I), where stable concentrations long-term are biologically desirable, for survival of the individual, insulin secretion needs to be regulated rapidly and precisely with secreted insulin cleared swiftly. Circulating insulin has a half-life of ~4 minutes9 and a duration of action (when given IV) of ~20 minutes. The half-life of C-peptide is somewhat longer, being near 10 minutes.9 So…why not measure “free” insulin? Indeed, we do measure free thyroxine (T4) routinely in assessing thyroid function.
Measuring free insulin
The problem with free insulin measurements is that these measurements are poorly standardized. The pre-analytical conditions can greatly affect this measurement.10 One paper concluded that samples for free insulin should be immediately treated with polyethylene glycol to remove antibody-bound insulin prior to analysis.11 It is highly unlikely that this is done in routine clinical laboratories when only reference laboratories measure free insulin. The type of precipitation method for the measurement of free insulin can also alter the free insulin concentration by a factor of two!12
Without standardization, we are reluctant to endorse free insulin measurements as clinically useful in the routine evaluation of persons with T1DM and hypoglycemia or hyperglycemia. Certainly total insulin measurements in insulin-treated patients with IA are uninformative. Measuring C-peptide should be helpful in assessing whether insulin is being secreted endogenously. This is because injected insulins lack C-peptide and, therefore, there is no exogenous C-peptide to serve as an immunogen to stimulate C-peptide antibodies. Thus, C-peptide measurements are technically and clinically valid. Last, it is important to note that, unlike free insulin, we do not measure free T4 by precipitation methods after the removal of the protein-bound T4. Such methods are not accurate for the measurement of free T4, nor are such methods reproducible.
Insulin autoantibodies
Can IAA ever cause hypoglycemia? The answer is yes: IAS is a real condition. In IAS, IAA develop that can release excessive quantities of insulin, raising the free insulin concentration and abruptly producing hypoglycemia. At other times, hyperglycemia may develop from inadequate levels of free insulin.
Insulin autoimmune syndrome
IAS is a rare condition (except possibly in Japan where it is reported to be the third-most common cause of hypoglycemia13). Between 1970 and 2007, there were 380 reported cases of IAS, and most cases were reported from Japan.14 In the almost 20 years that we have measured IAA (and our method for IAA also detects IA and does not distinguish IAA from IA), we are aware of only one regional case of IAS. [Note: In our laboratory IAA (and IA) are measured by the immunoprecipitation of patient serum with polyethylene glycol after a one-week incubation with added A14-monoiodinated insulin.]
Some authors define IAS as occurring only in non-insulin treated individuals.15 Generally, IAS is not associated with T1DM, although the concurrence of these disorders has been reported.16 More commonly, IAS is seen in association with Graves disease and/or the use of drugs that can include sulfur atoms such as methimazole, alfa-mercaptopropionyl glycine, glutathione, or alpha lipoic acid (marketed as an anti-aging/dieting supplement).17-20 Some investigators have reported insulin receptor autoantibodies in the serum of persons with the IAS.21 We are not aware of any commercial assays that measure insulin receptor autoantibodies.
Insulin antibodies
Can IA, which are acquired after treatment with insulin, cause hypoglycemia or hyperglycemia? While there are scattered case reports that IA can cause hypo- or hyperglycemia in insulin-treated patients,22-24 overall there is little if any evidence that IA alone routinely cause dysglycemia. This was the conclusion of a 2005 Cochrane review.25 Fifteen years earlier, researchers in Sweden reached the same conclusion.26 Still a third paper from Bucharest published in 1990 found no relationship between IA and hypoglycemia.27
Injected insulins
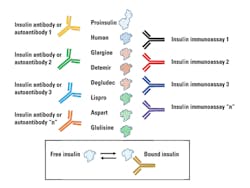
Further complicating insulin measurements in insulin-treated patients is the nature of the injected insulin (Table 1, Figure 1). For example, one insulin immunoassay only detects human insulin and does not detect recombinant-DNA-produced insulins with altered amino acid sequences. Therefore, the nature of the injected insulin and insulin specificity of the immunoassay must be known. There is also the potential complication of endogenous production (the patient’s “human” insulin) and human insulin of exogenous origin. If IA are not present, having separate immunoassays for insulin, proinsulin, and all of the sequence-modified insulins could be beneficial. There may be a need to measure insulin in persons with diabetes if an insulinoma is suspected. Insulinoma has rarely been reported in patients with diabetes.28,29 Possibly the earliest report of this is from 1958.30
Hypoglycemia in persons with diabetes
The most common causes of hypoglycemia in persons with T1DM are excess insulin doses, excessive exercise, and/or missed meals or snacks.31,32 If one accepts that free insulin measurements are not reliable, in persons with IA (representing the majority of persons treated with insulin injections), insulin measurements are not informative. Furthermore, if one is to measure insulin in insulin-treated patients, one may need unique insulin assays that can individually detect the different forms of insulin that are injected. As noted above, if patients do make their own insulin, the interpretation is further complicated. If there are IA, then the only assay of potential value is the C-peptide measurement, which assesses only endogenous insulin secretion.
Hyperglycemia in persons with diabetes
In the case of T1DM patients with poor metabolic control (i.e., an elevated HbA1c), if IA exist (as is the case in the majority of insulin-treated patients), the level of exogenous insulin cannot be assessed. If IA are absent and insulin levels are being measured to assess control, the critical issue is having insulin assays that are capable of detecting the various species of exogenous insulin. While insulin resistance can develop in persons treated with insulin, the most common cause of poor metabolic control is inadequate insulin (including missed doses of insulin), mistimed insulin administration, excess caloric intake, and/or inadequate exercise.
In summary, IA are extremely common in insulin-treated patients. Thankfully, IA only rarely cause hypo or hyperglycemia. Measuring insulin in insulin-treated patients is very complex. If IA are present, we do not recommend measuring insulin. Measurements of free insulin are troublesome and their reliability is questionable. If IA are absent, measuring insulin is complicated by the various species of insulin that are injected and the ability of the insulin assay to detect non-human insulins.
REFERENCES
- Winter WE, Schatz DA. Autoantibody Markers in Diabetes. Clin Chem. 2011;57(2):168-175.
- Winter WE, Pittman D. The clinical application of islet autoantibody testing for the diagnosis of autoimmune diabetes. MLO. 2013;45(10):16-26.
- Paiva ES, Pereira AE, Lombardi MT, et al.Insulin autoimmune syndrome (Hirata disease) as differential diagnosis in patients with hyperinsulinemic hypoglycemia. Pancreas. 2006;32(4):431-432.
- Sapin R. Insulin assays: previously known and new analytical features. Clin Lab. 2003;49(3-4):113-121.
- Hattori N, Duhita MR, Mukai A, Matsueda M, Shimatsu A. Development of insulin antibodies and changes in titers over a long-term period in patients with type 2 diabetes. Clin Chim Acta. 2014 Jun 10;433:135-138.
- Lauritano AA, Clements RS Jr, Bell D. Insulin antibodies in non-insulin-dependent diabetes mellitus: effect of treatment with semisynthetic human insulin. Clin Ther. 1989;11(2):268-277.
- Wredling R, Lins PE, Adamson U. Prevalence of anti-insulin antibodies and its relation to severe hypoglycaemia in insulin-treated diabetic patients. Scand J Clin Lab Invest. 1990;50(5):551-557.
- Mianowska B, Szadkowska A, Pietrzak I, et al. Immunogenicity of different brands of human insulin and rapid-acting insulin analogs in insulin-naïve children with type 1 diabetes. Pediatr Diabetes. 2011;12(2):78-84.
- Matthews DR, Rudenski AS, Burnett MA, Darling P, Turner RC. The half-life of endogenous insulin and C-peptide in man assessed by somatostatin suppression. Clin Endocrinol (Oxf). 1985;23(1):71-79.
- Rudkowski R, Antony G. The effect of immediate polyethylene glycol precipitation on free insulin measurements in diabetic patients with insulin antibodies. Diabetes. 1986;35(3):253-257.
- Arnqvist H, Olsson PO, von Schenck H. Free and total insulin as determined after precipitation with polyethylene glycol: analytical characteristics and effects of sample handling and storage. Clin Chem. 1987;33(1):93-96.
- Hwang DL, Barseghian G, Lev-Ran A. Determination of free-insulin in antibody containing sera: comparison of polyethylene glycol and Staphylococcus aureus cells. Horm Metab Res. 1985;17(11):595-597.
- Savas-Erdeve S, Yılmaz Agladioglu S, Onder A, et al. An uncommon cause of hypoglycemia: insulin autoimmune syndrome. Horm Res Paediatr. 2014;82(4):278-282.
- Uchigata Y, Hirata Y, Iwamoto Y. Drug-induced insulin autoimmune syndrome. Diabetes Res Clin Pract. 2009;83(1):e19-20.
- Wong SL, Priestman A, Holmes DT. Recurrent hypoglycemia from insulin autoimmune syndrome. J Gen Intern Med. 2014;29(1):250-254.
- Jassam N, Amin N, Holland P, et al. Analytical and clinical challenges in a patient with concurrent type 1 diabetes, subcutaneous insulin resistance and insulin autoimmune syndrome. Endocrinol Diabetes Metab Case Rep. 2014;2014:130086.
- Okazaki-Sakai S, Yoshimoto S, Yagi K, Wakasugi T, Takeda Y, Yamagishi M. Insulin autoimmune syndrome caused by an adhesive skin patch containing loxoprofen-sodium. Intern Med. 2013;52(21):2447-2451.
- Gullo D, Evans JL, Sortino G, Goldfine ID, Vigneri R. Insulin autoimmune syndrome (Hirata disease) in European Caucasians taking α-lipoic acid. Clin Endocrinol (Oxf). 2014;81(2):204-209.
- Zhao WC, Jin J, Zhang JZ. Insulin autoimmune syndrome induced by methimazole in a patient with Graves’ disease. J Endocrinol Invest. 2013;36(6):450-451.
- Gomez Cruz MJ, Jabbar M, Saini N, et al. Severe hypoglycemia secondary to methimazole-induced insulin autoimmune syndrome in a 16-year-old African American male. Pediatr Diabetes. 2012;13(8):652-655.
- Kim SW, Won HK, Seok H, et al. High prevalence of both anti-insulin and anti-insulin receptor antibodies in Korean patients with insulin autoimmune syndrome. Diabetes Res Clin Pract. 2012;98(2):e12-5.
- Koyama R, Nakanishi K, Kato M, Yamashita S, Kuwahara H, Katori H. Hypoglycemia and hyperglycemia due to insulin antibodies against therapeutic human insulin: treatment with double filtration plasmapheresis and prednisolone. Am J Med Sci. 2005;329(5):259-264.
- Ishizuka T, Ogawa S, Mori T, et al. Characteristics of the antibodies of two patients who developed daytime hyperglycemia and morning hypoglycemia because of insulin antibodies. Diabetes Res Clin Pract. 2009;84(2):e21-3.
- Segal T, Webb E, Viner R, Pusey C, Wild G, Allgrove J. Severe insulin resistance secondary to insulin antibodies: successful treatment with the immunosuppressant MMF. Pediatr Diabetes. 2008;9(3 Pt 1):250-254.
- Richter B, Neises G. “Human” insulin versus animal insulin in people with diabetes mellitus. Cochrane Database Syst Rev. 2005 Jan 25;(1):CD003816.
- Wredling R, Lins PE, Adamson U. Prevalence of anti-insulin antibodies and its relation to severe hypoglycaemia in insulin-treated diabetic patients. Scand J Clin Lab Invest. 1990;50(5):551-557.
- Ionescu-Tîrgovis¸te C, Mirodon Z, Paterache E, Chet¸a D, Mincu I. No relationship between insulin antibodies and hypoglycemia in insulin-treated diabetic patients. Rom J Intern Med. 1991;29(3-4):189-198.
- Lablanche S, Chobert-Bakouline M, Risse O, Laverrière MH, Chabre O, Benhamou PY. Malignant insulinoma may arise during the course of type 1 diabetes mellitus: a case report. Diabetes Metab. 2015;41(3):258-261.
- Ghafoori S, Lankarani M. Insulinoma in a patient with type 2 diabetes mellitus. Acta Med Iran. 2015;53(5):317-319.
- Gittler RD, Zucker G, Eisenger R, Stoller N. Amelioration of diabetes mellitus by an insulinoma. N Engl J Med. 1958 May 8;258(19):932-935.
- Unger J, Parkin C. Hypoglycemia in insulin-treated diabetes: a case for increased vigilance. Postgrad Med. 2011;123(4):81-91.
- Santiago JV, Levandoski LA, Bubb JA, White NH. Definitions, causes, and risk factors for hypoglycemia in insulin-dependent diabetes. Curr Ther Endocrinol Metab. 1997;6:447-453.
William E. Winter, MD, DABCC, FACB, FCAP, is Professor of Pathology and Pediatrics at the University of Florida. Dr. Winter directs the Endocrine Autoantibody Laboratory that is part of the University of Florida Health Pathology Laboratories (UFHPL).
David Pittman serves as the manager of the University of Florida Health Pathology Laboratories (UFHPL), Endocrine Autoantibody Laboratory. Mr. Pittman is responsible for both the clinical and research activities of the laboratory unit including the Islet Cell Autoantibody (ICA) Core Laboratory for the Type 1 Diabetes TrialNet study, and thyroid autoantibody and TSH testing for the TEDDY study (The Environmental Determinants of Diabetes in Youth).
Laura M. Jacobsen, MD, is currently a second-year pediatric endocrinology fellow at the University of Florida. Dr. Jacobsen completed her medical school training at the University of Florida and her pediatric residency at the University of North Carolina in Chapel Hill.