Too much A2 – but does the patient really have beta thalassemia trait?
In routine laboratory practice, the diagnosis of beta thalassemia trait is usually made by characteristic findings in the hemoglobin evaluation and the red blood cell count and indices. In particular, the percentage of hemoglobin (Hb) A2 is elevated, while the red cell mean corpuscular volume (MCV) and/or mean corpuscular hemoglobin (MCH) are decreased. The red blood cell count is usually normal or increased, but may be decreased if the patient has other causes of anemia.
Beta thalassemia trait (also called beta thalassemia minor or beta thalassemia carrier state) is a benign, heterozygous condition that can be distinguished from the more severe beta thalassemia syndromes (intermedia and major) by clinical and laboratory features. Beta thalassemia intermedia and major are associated with increasing severity of anemia, transfusion dependence, and splenomegaly, while these features are absent in beta thalassemia trait. In severe beta thalassemia, the level of fetal hemoglobin (Hb F) is markedly increased due to the absence of Hb A, and the amount of Hb A2 may not be increased as in beta thalassemia trait. So the laboratory diagnosis of beta thalassemia trait should be relatively straightforward, correct?
Not always. The following two cases illustrate common situations that can complicate the diagnosis.
Case 1: competing conditions
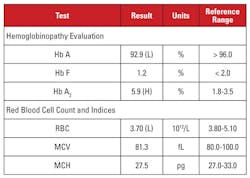
The patient was a 28 year-old African-American female who was positive for HIV-1 infection and was undergoing highly active antiretroviral therapy (HAART) with a dolutegravir/abacavir/lamivudine regimen. Laboratory findings are shown in Table 1.
This case presented an interesting situation. Although the Hb A2 level was significantly elevated, the corresponding hemogram did not demonstrate microcytosis or hypochromia. When laboratory features are discordant for a diagnosis of beta thalassemia trait, it is useful to consider other conditions that affect the Hb A2 level and red blood cell indices. In this case, an important clue was found in the patient’s clinical history.
Hb A2 levels tend to increase in conditions that delay nuclear maturation of red cell precursors. These conditions are also associated with increased MCV.1,2 The most common cause of this phenomenon is megaloblastic anemia due to folate and/or vitamin B12 deficiency. However, several medications that inhibit nucleic acid synthesis have a similar effect, including the class of anti-HIV medications called nucleoside reverse transcriptase inhibitors (NRTIs). Lamivudine in this patient’s HAART regimen is an NRTI medication.
So should we conclude that this patient’s high Hb A2 level was due to the anti-HIV medication, and the patient did not have beta thalassemia trait? Not so fast! In general, increases in Hb A2 due to NRTI (or megaloblastic anemia) are less than that seen in beta thalassemia trait.1,2 In addition, the increases tend to be proportional to overall effects of the drug, which can be approximated by increases in MCV.1,2 In many patients on NRTI therapy, the MCV is markedly high (often greater than 120 fL), whereas in our patient, the MCV was toward the low end of the reference range.
Therefore, we were not convinced that the patient’s very high Hb A2 level was due solely to lamivudine therapy. We suspected that the patient had competing conditions that increased and decreased the MCV (i.e., beta thalassemia trait and lamivudine therapy, respectively), with both contributing to a high Hb A2 level. We reported to the ordering physician that beta thalassemia trait was likely, and could be confirmed by beta globin mutation analysis. Subsequent gene sequencing revealed a heterozygous beta globin mutation associated with beta-zero thalassemia.
Case 2: ethnicity factor
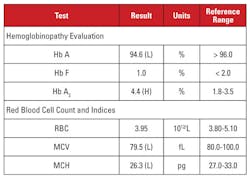
The patient was a 35 year-old pregnant Vietnamese female who was being screened for hemoglobinopathy. Laboratory findings are shown in Table 2.
Again, the high Hb A2 level was discordant with minimal or absent microcytosis and hypochromia. In this case, we were able to rule out common confounding factors such as folate, vitamin B12 or iron deficiency, thyroid dysfunction, or medication effects. Based on the patient’s ethnicity, we suspected another important cause of discordance: beta thalassemia trait co-inherited with alpha thalassemia minor. When a patient inherits both of these conditions, the MCV and MCH often normalize because alpha and beta globin chains are present in relatively balanced amounts in developing red blood cells.2
We recommended genetic analyses to test for alpha and beta thalassemia mutations, in order to fully assess the risk to the pregnant mother and the fetus. Subsequent beta globin sequencing revealed a heterozygous beta-plus thalassemia mutation, while analysis for alpha globin deletions revealed a heterozygous Southeast Asian (SEA) two-gene deletion, consistent with –/αα alpha thalassemia minor. Based on these findings, genetic analysis of the father was indicated to assess the risk to the fetus of inheriting a clinically severe thalassemia syndrome.
Alpha and beta thalassemias
Thalassemias are among the most common inherited disorders in mankind. They are highly prevalent where malaria has been endemic, and are now common in every part of the world due to migration of human populations. Thalassemias are caused by mutations that reduce globin gene expression in red blood cell precursors. The disorders are classified by the globin genes that are mutated (for example, alpha versus beta) and by the severity of disease, which is related to whether mutations are inherited in a heterozygous or a homozygous/compound heterozygous fashion. The major adult form of hemoglobin is Hb A, a tetramer of two alpha and two beta globin chains. In alpha thalassemia, alpha globin expression is deficient and there is a corresponding excess of beta globin chains.
This pattern is reversed in beta thalassemia. In the setting of globin chain deficiency and imbalance, red cell hemoglobin content is decreased, resulting in microcytosis and hypochromia. In addition, excess alpha or beta globin chains form unstable tetramers that cause hemolysis. Alpha and beta thalassemia are distinguished by the amount of the minor adult hemoglobin Hb A2, a tetramer of two alpha and two delta globin chains. Hb A2 is increased in beta thalassemia because the relative lack of beta globin allows more delta chains to be incorporated into hemoglobin.
Beta thalassemia is caused by mutations in the beta globin gene locus on chromosome 11.3,4 Most mutations are small nucleotide substitutions, insertions or deletions, although large deletions are identified in rare cases. Depending on the mutation, beta globin expression is reduced partially (beta-plus thalassemia) or completely (beta-zero thalassemia). Beta thalassemia trait is caused by a heterozygous mutation. This condition is asymptomatic, and is characterized by increased Hb A2, red cell microcytosis, and no significant hemolytic anemia. In contrast, beta thalassemia major (Cooley’s anemia) is caused by homozygous beta-zero mutations. Hemoglobin evaluation reveals a predominance of Hb F, absent Hb A, and normal or increased Hb A2. Beta thalassemia major is characterized by severe, transfusion-dependent hemolytic anemia, with resulting splenomegaly and bone deformities. Recurrent transfusion leads to iron overload, which is a principal cause of morbidity and mortality. Beta thalassemia intermedia is clinically heterogeneous, with most symptoms related to moderate hemolytic anemia. Transfusion dependence is uncommon because beta globin expression is not absent. Due to the large number of mutations associated with beta thalassemia, genetic diagnosis typically requires gene sequencing. The beta thalassemia syndromes are summarized in Table 3.
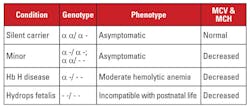
Alpha thalassemia is caused by mutations in the alpha globin gene locus on chromosome 16.5 Alpha globin genes are duplicated on chromosome 16, so a normal individual has four copies. The most common mutations are large deletions that affect one or both genes on the locus. A fairly limited number of deletions have been described, with prevalence in different populations; for example, large mutations that delete both alpha genes are very common in people of Southeast Asian ethnicity. These mutations are also prevalent in Mediterranean populations, but are rare in people of African ethnicity. In contrast, deletions of single alpha globin genes have a widespread distribution. The loss of one of the four alpha globin genes is referred to as a silent carrier state.
This condition is asymptomatic and is usually associated with normal red blood cell indices. The loss of two alpha globin genes is termed alpha thalassemia minor. This condition can occur due to homozygous one-gene deletions or heterozygous two-gene deletions. The red cell count and indices are indistinguishable from beta thalassemia trait, but the Hb A2 level is normal. The loss of three alpha genes is termed Hb H disease. (Hb H is a tetramer of beta globin chains.) Hb H disease is typically a moderate hemolytic anemia associated with splenomegaly and bone changes. Transfusion dependence is uncommon.
The loss of all four alpha genes is termed Hb Barts hydrops fetalis (Hb Barts is a tetramer of fetal gamma globin chains). This condition is incompatible with postnatal life. Hb H and Hb Barts can be identified in hemoglobin evaluations and play a role in postnatal diagnosis of alpha thalassemia.5
Genetic diagnosis is usually accomplished by gap-polymerase chain reaction (PCR) assays that target the common deletions. Alpha globin gene sequencing is also available to detect rare mutations. The alpha thalassemia syndromes are summarized in Table 4.
When genetic testing is needed
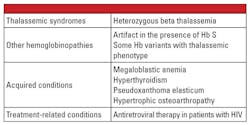
In conclusion, the heterozygous forms of alpha and beta thalassemia are very common and are identified frequently in the clinical laboratory. Hemoglobin evaluation and other routine clinical and laboratory data are usually sufficient for diagnosis. Genetic testing is not necessary in most cases, but can be critically important in the context of family planning. If thalassemia mutations are identified in both parents, there may be significant risk to the fetus for inheriting severe thalassemia. In the case of hydrops fetalis, fetal demise is likely and there is significant risk to the mother for complications of pregnancy.5 Therefore, it is important to be aware of variables that complicate the laboratory diagnosis of thalassemias, and recognize when confirmatory genetic testing is indicated. For example, as shown in the first case report, the diagnosis of beta thalassemia trait can be challenging if there are co-existing conditions that affect the level of Hb A2. Factors that are known to increase Hb A2 are listed in Table 5.6
REFERENCES
- Steinberg MH, Forget BG, Higgs DR, et al. Disorders of Hemoglobin: Genetics, Pathophysiology Clinical Management. Cambridge: Cambridge
University Press. 2009. - Steinberg MH, Rodgers GP. HbA2: biology, clinical relevance and a possible target for ameliorating sickle cell disease. Br J Haematol. 2015;170(6):781-787.
- Cao A, Galanello R. Beta-thalassemia. Genet Med. 2010;12(2):61-76.
- Origa R. β-Thalassemia. Genet Med. 2017;19(6):609-619.
- Galanello R, Cao A. Gene test review. Alpha-thalassemia. Genet Med. 2011;13(2):83-88.
- Stephens AD, Angastiniotis M, Baysal E, et al. ICSH recommendations for the measurement of haemoglobin A2. Int J Lab Hematol. 2012;34(1):1-13.
Andrew N. Young , MD, PhD, serves as Medical Director for Quest Diagnostics.
Thomas E. Burgess, PhD, DABCC, FACB, serves as Technical Director for Quest Diagnostics’ Southeast business based in Atlanta, GA.