CONTINUING EDUCATION
To earn CEUs, visit www.mlo-online.com under the CE Tests tab.LEARNING OBJECTIVES
1. Describe personalized medicine and the healthcare benefits it can provide.
2. List and discuss human genetic variations and their roles in pharmacogenomics.
3. Discuss pharmacogenomics in drug development as it relates to drug metabolism.
4. Discuss the role pharmacogenomics has played in developing guidelines and clinical applications of drugs.
Personalized medicine, often called precision medicine, is a medical practice in which patients are prescribed medications that are appropriate to them, based on their genetic, environmental, and lifestyle factors. It is an approach, enabled by molecular diagnostics, that contrasts with the traditional practice of treating all patients with the same disease with the same drug and with the same dosage.
In fact, the practice is not really new. It was followed 2,500 years ago in ancient Greece by Hippocrates, the “Father of Western Medicine.” As an interesting article by Sykiotis et al1 points out, Hippocrates believed in the individuality of disease and the necessity of giving “different [drugs] to different patients.” He evaluated factors like a person’s constitution, age, and physique, as well as the time of year, to decide how to, as it were, prescribe.
Now we know that variations among individuals are due to differences in their genetic make-up. It is known that not all patients respond to the same drug in the same way. In the United States, adverse drug reaction (ADR) is the fourth leading cause of death, and it is estimated that prescription drugs are responsible for 2.74 million ADRs and 128,000 deaths annually.2 ADRs cost $136 billion yearly—more than the total costs of cardiovascular and diabetes care—and cause one out of five injuries or deaths per year tohospitalized patients.3-5
Pharmacogenomics and pharmacogenetics
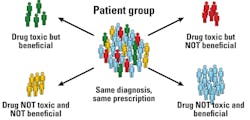
Pharmacogenomics is the study that deals with the relationship between genomic variations and their effect on drugs. Though the terms pharmacogenomics (PGx) and pharmacogenetics (PGt) are often used interchangeably, pharmacogenetics usually refers to the effect of a single gene on drug response. Figure 1 illustrates the inter-individual variability with drug response.
Pharmacogenomics plays two major roles in precision medicine. First, it guides pharmaceutical companies in drug discovery and development. Second, it guides physicians in selecting the right drug for patients based on their genetic make-up, in avoiding ADR, and in maximizing drug efficacy by prescribing the right dose.
Genetic variations
The Human Genome Project (HGP), concluded in April 2003, revealed that humans have about 20,500 genes and that 99.5 percent of the genes are similar.6 The remaining 0.5 percent are variations that are responsible for the individual’s eye color, blood group, predisposition toward particular diseases, etc. The most common type of DNA sequence variation found in the human genome is the single nucleotide polymorphism (SNP, pronounced “snip”). Another type of variation, called structural variations (SV), are deletions, insertions, tandem repeats, inversions, and copy number variations (CNV). There are approximately 11 million SNPs in the human genome, with an average of one every 1,300 base pairs. SNPs act as biological markers and determine an individual’s response to certain drugs, susceptibility to environmental factors such as toxins, and risk of developing disease.
Genetic differences among individuals can affect virtually all aspects of a disease and its treatment.7 Genetic variations can affect disease management with regard to the following:
- the rate of disease occurrence
- the risk of disease progression or recurrence
- the drug or drug class most likely to provide benefit
- the therapeutic dose
- the nature and extent of beneficial responses to treatment
- the likelihood of drug toxicity.
Genetic variations relevant to drug development5 include:
- genes relevant to the drug’s pharmacokinetics (absorption, distribution, metabolism [including formation of active metabolites], and excretion)
- genes that code for intended or unintended drug targets and other pathways related to the drug’s pharmacologic effect
- genes that can predispose to toxicities such as immune reactions
- genes that influence disease susceptibility or progression.
All of these genetic factors can affect the benefit–risk drug profile.
Pharmacogenomics in drug development
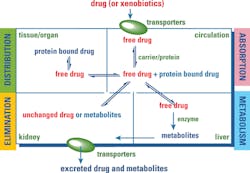
To successfully develop personalized dosing regimens for patients, an understanding of pharmacokinetics (PK), pharmacodynamics (PD), and PGx is important. Every drug entering the body goes through the process of absorption, distribution, metabolism, and excretion (ADME). The sum of all these processes is PK, which determines how much of the drug is needed to reach the site of action for effective therapeutic outcome. Figure 2 depicts the process of PK.
The drug also causes physiological and biochemical changes in the body. PD is the mechanism of action of the drug and its effect on the body. It determines how well the target cells, such as heart tissue or neurons, respond to the drug. The drug manufacturer determines the intricate balance between the PK and PD so that the drug has the maximum intended effect and the minimal potential adverse effect on the patient.
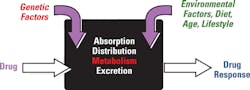
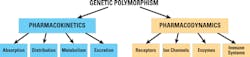
The innate genetic polymorphism in an individual can cause a shift in the balance of PK and PD, resulting in an alteration in the way the body and the drug (or its metabolites) interact with each other. PGx holds the promise that individuals can be given personalized medications guided by information on PGt testing, and the individual’s environment, diet, age, lifestyle, and current state of health. Figure 3 illustrates the factors responsible for variation in drug response.
Use of PGx in improving PK and PD balance
Forty percent of the failures in drug development are attributed to PK issues.8 There are more than 170 genes known or suspected to have a role in drug disposition, of which more than half are known to be polymorphic.9 Pharmacokinetic processes show inter-individual variability and are subject to both environmental and host-determined influences such as physiological and genetic factors. Genetic polymorphisms are mostly responsible for inter-individual variability and can have the following consequences in any of the ADME:9
- altered absorption/clearance
- differences in formation of active metabolites
- changes in drug interactions
- ethnic variation related to drug response.
PK/PGx in absorption of drugs9
Absorption is the movement of the drug from its site of administration into the bloodstream. It’s a complex process that involves several membrane-bound drug transporters, such as P-glycoprotein (P-gp, MDR1) and multidrug resistance (MDR) transporters, encoded by the ABC genes. The gene ABCB1, which codes for P-gp, has more than 50 SNPs, which vary in frequency based on ethnicity. These mechanisms have a bearing on the ultimate bioavailability of the drug. Bioavailability is the fraction of the drug that reaches the bloodstream or the site of action after it is administered.
PK/PGx in distribution of drugs9
Following administration, the drug is distributed into all of the body compartments and tissues that it is able to enter based on its physical-chemical properties. The drug is distributed into an imaginary volume, called volume of distribution (Vd), which is primarily dependent on physiological parameters such as body mass index and fat deposits. Vd may be dependent on PGx for distribution to certain body compartments such as the brain across the blood-brain barrier and breast milk, which are dependent on transporter genes like ABC. Overexpression of these genes can result in drug resistance. Polymorphism in genes ABCB1 and SLCO1B1 have also been shown to affect drug distribution.
PK/PGx in metabolism of drugs9
Drug metabolism is the metabolic breakdown of drugs, usually through specialized enzymatic systems. Most drug metabolism occurs in the liver and intestine. Drug metabolism is divided into three phases:10
Phase I metabolism involves:
- oxidation (via cytochrome P450), reduction, and hydrolysis reactions
- conversion of a parent drug to more polar (water soluble) active metabolites by unmasking or inserting a polar functional group (-OH, -SH, -NH2).
Drugs metabolized via phase I reactions have longer half-lives.
Phase II metabolism involves:
- glucuronidation, acetylation, and sulfation reactions
- “conjugation reactions” that increase water solubility of a drug with a polar moiety glucuronate, acetate, and sulfate, respectively11
- conversion of a parent drug to more polar (water soluble) inactive metabolites by conjugation of subgroups to -OH, -SH, -NH2 functional groups in the drug
- drugs metabolized via phase II reactions that are excreted via the kidney.
Patients deficient in acetylation capacity (slow acetylators) may have prolonged or toxic responses to normal doses of certain drugs because of decreased rates of metabolism.
Phase III metabolism involves:
- further modification of the conjugated drug and excretion
- a detoxification process and transportation of the conjugates against a concentration gradient out of the cell into the interstitial space between cells
- the conjugated drug entering the capillary system and then the main bloodstream, and filtration by the kidneys.
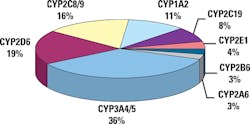
The highest levels of polymorphism are found in genes involved in drug metabolism, especially the cytochrome (CYP) 450 genes. These account for 80 percent of current PGx drug labeling requirements by the FDA. There are approximately 50 CYP 450 genes: 49 genes and one pseudogene. There are numerous isoforms of CYP450. Isoforms are CYP enzyme variants that have derived from one particular gene. CYP isoforms are classified into families and subfamilies. CYP families are genes that have at least 40 percent sequence homology. Members of a subfamily must have at least 55 percent sequence homology. Only about a dozen enzymes belonging to the 1, 2, and 3 CYP-families are responsible for the metabolism of the majority of drugs and other xenobiotics.
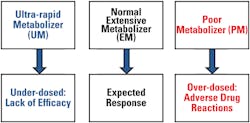
Genetic polymorphisms in drug metabolism genes are expressed as differing phenotypes. Figure 4 shows the different phenotypes for polymorphisms in drug metabolism genes. The major CYP 450 genes involved in drug metabolism and the proportions of drugs metabolized by them are provided in Figure 5.10,12
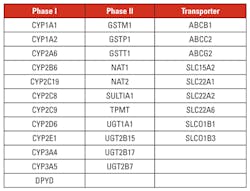
A group of 32 genes has been designated by the PharmaADME Working Group as the core ADME genes for pharmacogenomics study for drug development. ADME genes are provided in Table 1.
With respect to pharmacodynamics, knowledge of pharmacogenomics helps select the right targets for the molecular action of a drug. The target may be the cell surface (e.g., a receptor), an ion channel, or an intracellular target (e.g., an enzyme or regulatory protein). Figure 6 captures two major determinants of drug development—PK and PD—which are greatly influenced by genetic polymorphisms.13
Use of pharmacogenomics principles have become an integral part of early drug discovery processes. Their application continues during preclinical and clinical drug development.14
Regulatory guidelines
Over the last decade, the FDA has been aggressive in providing genetic labeling on new drugs, and also updating product labels for a number of existing therapies, such as the blood thinner warfarin and 6-mercaptopurine. At present some 14015 drugs have PGt information in their FDA product label. A current listing of all drugs with pharmacogenetics labeling can be found in the FDA Table of Pharmacogenomics Biomarkers in Drug Labels. Pharmacogenetics information in product labels ranges from boxed warnings, the highest level of warning, to information in the clinical pharmacology section.
Clinical applications of PGx/PGt
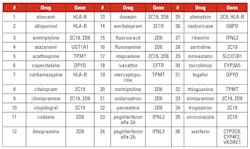
The Clinical Pharmacogenetics Implementation Consortium (CPIC), formed in 2009, sets guidelines on drug therapy based on pharmacogenetics information.16 As of March 2017, 36 CPIC guidelines have been published. The list of drugs and the genes for which the guideline exists are provided in Table 2.
Production of a number of other guidelines is ongoing, and a listing of other guidelines on pharmacogenomics can be found on The Pharmacogenomics Knowledgebase.17
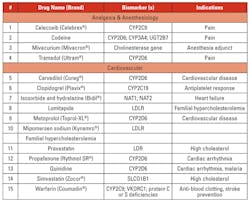
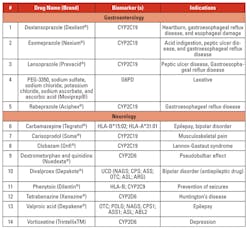
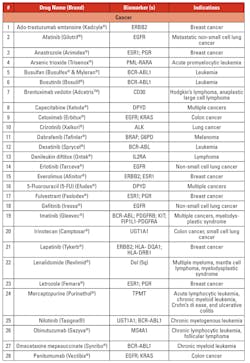
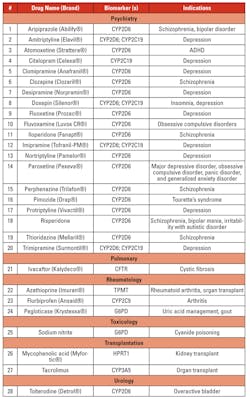
Personalized/precision medicine is being used in several disease conditions to determine treatment options based on genetic mutations. Tables 3a, 3b, 3c, and 3d list the gene mutations for some such drugs and relevant biomarkers.18
Pharmacogenetics also plays an important role in polypharmacy with elderly patients by averting ADRs from drug-drug interactions.19,20
PGt testing in the clinical setting
Using pharmacogenetics testing data to select the appropriate drug and dosage for the patient can have considerable benefits for all stakeholders. The patient can learn about his or her predisposition to a disease and take appropriate precautions to prevent onset, receive personalized/precision medication, and recover sooner without episodes of adverse drug events, and can save time and unnecessary expenses by avoiding ADRs. The physician can provide better patient care and retain satisfied—and healthier—patients. Payers can help to avoid unnecessary healthcare costs incurred due to ADRs, inpatient hospital stays, etc. The following illustrate the economic benefit of pharmacogenetics:
- A Mayo Clinic study involving 3,600 subjects showed that hospitalization of heart patients was reduced by 30 percent when doctors were made aware of the pharmacogenetics data prior to treatment with warfarin.21
- Breast cancer therapy guided by a commercially available PGt test was able to achieve a cost savings of $2,256 per patient, as a result of the reduction in the use of chemotherapy.22
- When treatment with panitumumab (Vectibix) or cetuximab (Erbitux) was limited to patients with metastatic colorectal cancer whose KRAS gene was not mutated, an annual savings of $604 million among all patients was achieved.23
The future is here
Pharmacogenomics and precision medicine are the future of healthcare. The U.S. Congress approved a $2 billion increase in NIH funding for the Precision Medicine Initiative (PMI) in 2016.24 The long-term goals of the PMI focus on bringing precision medicine to all areas of health and healthcare on a large scale. To this end, the NIH plans to launch a study, known as AllofUs, which will involve a cohort of at least one million volunteers from around the U.S. Participants will provide genetic data, biological samples, and other information about their health. Researchers will use these data to study a large range of diseases, with the goals of better predicting disease risk, understanding disease occurence, and finding improved diagnosis and treatment strategies.25
To reap the full benefits of pharmacogenomics, genetic variability considerations should not be included only in the early phases of drug discovery, but should also be used in subsequent phases of drug development. That would help pharma companies to avoid losses due to drug failures after substantial expenditure has already been invested on the drug development.
The potential advantages of personalized/precision medicine, based on the knowledge of pharmacogenomics, are profound. There is no doubt it will eventually become the standard of care.
REFERENCES
- Sykiotis GP, Kalliolians GD, Papavassiliou AG. Pharmacogenetic principles in the Hippocratic writings. J Clin Pharmacol. 2005;45(11):1218–1220.
- Light DW. New prescription drugs: a major health risk with few offsetting advantages. Harvard University. Edmond J. Safra Center for Ethics. June 27, 2014.
https://ethics.harvard.edu/blog/new-prescription-drugs-major-health-risk-few-offsetting-advantages. - Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995;155(18)1949-1956.
- Leape LL, Brennan TA, et al. NEJM. The nature of adverse events in hospitalized patients—results of the Harvard Medical Practice Study II. 1991;324(6)377-384.
- Classen DC, Pestotnik SL, Evans SR, et al. Adverse drug events in hospitalized patients: excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277(4):301-306.
- National Institutes of Health. National Human Genome Research Institute. An overview of the Human Genome Project. https://www.genome.gov/12011238/5.
- HHS. FDA. CDER. BER. CDRH. Guidance for industry—Clinical pharmacogenomics: premarket evaluation in early-stage clinical studies and recommendations for labeling. January 2013. https://www.fda.gov/downloads/Drug /GuidanceComplianceRegulatoryInformation/Guidances/UCM337169.pdf.
- Kubinyi H. Nature Reviews, Drug Discovery. 2003;2:665-668.
- Mifsud J, Maliepaard M. “Bringing the magic bullet closer to reality” in Preventive and Predictive Genetics: Towards Personalised Medicine, eds. Grech G,
Grossman I. - Wikipedia. Drug metabolism. https://en.wikipedia.org/wiki/Drug_metabolism.
- Jancova P, Siller M. “Phase 2 drug metabolism” in Pharmacology, Toxicology and Pharmaceutical Science, Paxton J, ed. https://www.intechopen.com/books/topics-on-drug-metabolism/phase-ii-drug-metabolism.
- Wikimedia. Untitled chart. https://upload.wikimedia.org/wikipedia/commons/0/08/Proportion_of_drugs_metabolized_by_different_CYPs.png.
- Adams J. Pharmacogenomics and personalized medicine. Nature Education, 2008;1(1):194.
- Higgins G. “The role of pharmacogenomics (PGx) in drug discovery” in Drug Discovery: Practices, Processes, and Perspectives, eds. Li JJ, Corey EJ.
- U.S. Food and Drug Administration. Table of pharmacogenomic biomarkers in drug labels. https://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm083378.htm.
- Relling MV, Klein TE. CPIC: Clinical pharmacogenetics implementation consortium of the pharmacogenomics research network. Clin Pharmacol Ther. 2011;89(3):464-467.
- PharmGKB. Dosing guidelines. https://www.pharmgkb.org/view/dosing-guidelines.do?source=CPIC.
- Personalized Medicine Coalition. The personalized medicine report. 2017—opportunity, challenges, and the future. Appendix. http://www.personalizedmedicinecoalition.org/Userfiles/PMC-Corporate/file/The-Personalized-Medicine-Report1.pdf.
- Finkelstein J, Friedman C, Hripcsak G, Cabrera M.Potential utility of precision medicine for older adults with polypharmacy: a case series study. Pharmgenomics Pers Med. 2016;15(9):31–45. doi: 10.2147/PGPM.S101474.
- Sreter KB, Barisic B, Popovic-Grle S. Pharmacogenomics and tailored polypharmacy: an 80-year-old lady with rosuvastatin-associated rhabdomyolysis and
maprotiline-related Ogilvie’s syndrome. Int J Clin Pharmacol Ther. 2017;55(5):442-448. - Epstein RS, Moyer TP, Aubert RE, et al. Warfarin genotyping reduces hospitalization rates. Results from the MM-WES. J Amer Col Cardiol. 2010;55(25):2804-2812.
- Genomic Health. Validity assessment of Oncotype Dx breast cancer assay economic analyses. http://breast-cancer.oncotypedx.com/en-US/Managed-Care/Health-Economics/Validity-Assessment.aspx.
- Carlson B, KRAS testing: optimizing cancer therapy. Biotechnol Healthcare. 2009;6(5):7-9.
- Kaiser J. Senate panel approves $2 billion raise for NIH in 2016. Science. June 23, 2015. http://www.sciencemag.org/news/2015/06/senate-panel-approves-2-billion-raise-nih-2016.
- NIH U.S. National Library of Medicine. Genetics Home Reference. https://ghr.nlm.nih.gov/.
Rajasri Chandra, MS, MBA, serves as Product Manager for molecular diagnostics provider AutoGenomics, Inc., based in Carlsbad, CA.